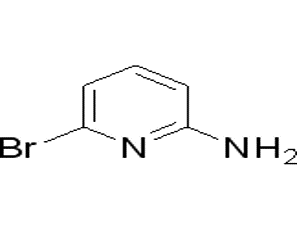
2-Amino-6-bromopyridine(CAS# 19798-81-3)
Risk and Safety
| Risk Codes | R36/37/38 – Irritating to eyes, respiratory system and skin. R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 – Wear suitable protective clothing. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. |
| WGK Germany | 3 |
| HS Code | 29333999 |
| Hazard Class | IRRITANT |
2-Amino-6-bromopyridine(CAS# 19798-81-3) Information
| Overview | 2-amino substituted nitrogen-containing six-membered heterocyclic compounds have important applications in the chemical industry, such as 2-amino -6-Bromopyridine is one of the important structures in synthetic drugs and agricultural chemical molecules, and is widely used in the synthesis of natural products, drugs, luminescent materials and various fine chemicals. |
| Application | 2-amino substituted nitrogen-containing six-membed heterocyclic compounds have important applications in the chemical industry, such as 2-amino -6-Bromopyridine is one of the important structures in synthetic drugs and agricultural chemical molecules, and is widely used in the synthesis of natural products, drugs, luminescent materials and various fine chemicals. |
| Preparation | Preparation of 2-amino-6-bromopyridine: Add 2-fluoro-6-bromo-pyridine (1mmol), pentamidine hydrochloride (2mmol), sodium tert-butoxide (3mmol),HO(0.5mL) and diethylene glycol dimethyl ether (2.5mL) in a 25 ml reaction tube. The reaction was carried out at 150 ℃ for 24 hours. After the reaction was completed, it was cooled to room temperature. Add 10mL of ethyl acetate to quench the reaction, add 6mL of saturated salt water to wash, separate the organic phase, then extract the aqueous phase with ethyl acetate for 3 times (the dosage of ethyl acetate each time is 6mL) and combine the organic phase, add anhydrous sodium sulfate to dry, remove the solvent including organic solvent and inorganic solvent by vacuum distillation, and then separate the organic solvent by column chromatography to obtain the target product 2-amino-6-bromopyridine with a yield of 93%. |
| use | pharmaceutical intermediates. for the efficient synthesis of 7-azafindole in one pot; for the synthesis of anti-HIV drugs |
Write your message here and send it to us