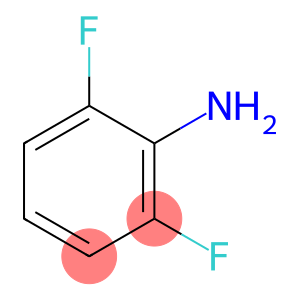
2-6-Difluoroaniline(CAS#5509-65-9)
| Risk Codes | R10 – Flammable R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S36 – Wear suitable protective clothing. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S23 – Do not breathe vapour. S16 – Keep away from sources of ignition. S16/23/26/36/37/39 - S24/25 – Avoid contact with skin and eyes. |
| UN IDs | UN 1993 3/PG 3 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 8-10-23 |
| HS Code | 29214210 |
| Hazard Note | Irritant |
| Hazard Class | 3 |
| Packing Group | III |
Introduction
2,6-Difluoroaniline is an organic compound. It is a white crystalline solid that is insoluble in water at room temperature.
The following are some of the properties and uses of 2,6-difluoroaniline:
1. 2,6-Difluoroaniline is an aromatic amine compound with a strong amine odor.
2. It is a strong electron donor that can be used as a component of conductor materials.
4. It is also commonly used as a catalyst or reagent in organic synthesis reactions.
Method for preparing 2,6-difluoroaniline:
A commonly used synthesis method is obtained by the reaction of aniline and hydrogen fluoride. First, aniline is reacted with hydrogen fluoride in an appropriate solvent, and the product is purified after the reaction to obtain 2,6-difluoroaniline.
Safety information of 2,6-difluoroaniline:
1. 2,6-Difluoroaniline is a harmful substance, irritating and corrosive. Precautions should be taken when in contact with skin, eyes, or inhalation.
2. Appropriate personal protective equipment should be used during operation, including chemical goggles, gloves and protective clothing, etc.
3. When mixed with other compounds, toxic vapors, gases, or fumes may be produced and need to be operated in a well-ventilated environment.
4. Before handling 2,6-difluoroaniline or its related compounds, the relevant safety operating procedures and guidelines should be understood and followed.