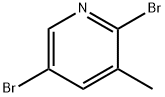
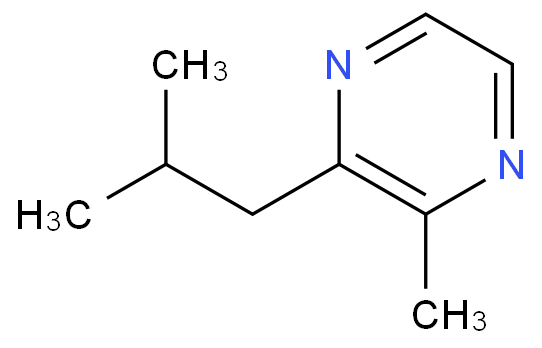
2 5-Dibromo-3-methylpyridine(CAS# 3430-18-0)
| Risk Codes | R22 – Harmful if swallowed R36/38 – Irritating to eyes and skin. R36/37/38 – Irritating to eyes, respiratory system and skin. R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S36 – Wear suitable protective clothing. |
| UN IDs | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| HS Code | 29333990 |
| Hazard Class | 6.1 |
| Packing Group | III |
Introduction
2,5-Dibromo-3-trimethylpyridine is an organic compound. The following is an introduction to some of its properties, uses, manufacturing methods and safety information:
Quality:
- Appearance: 2,5-Dibromo-3-trimethylpyridine is a colorless to pale yellow liquid.
- Solubility: It can be dissolved in many organic solvents, such as ethanol, acetone, and dimethyl sulfoxide.
- Stability: It is relatively stable to light and heat, but decomposition can occur under strong alkaline conditions.
Use:
- As a catalyst: 2,5-dibromo-3-trimethylpyridine can be used as a brominating agent to catalyze some organic reactions, such as nucleophilic substitution, oxidation, and condensation.
- Organic synthesis: It can be used as an intermediate in the synthesis of organic compounds, especially for compounds containing ketone or aldehyde groups.
- Photosensitive dyes: It can also be used in the preparation of photosensitive dyes.
Method:
In general, 2,5-dibromo-3-trimethylpyridine can be prepared by bromination reaction with bromine as the reactant in the reaction system of trimethylpyridine. Reaction conditions can be determined on a case-by-case basis, but care should be taken for safe operation.
Safety Information:
- 2,5-Dibromo-3-trimethylpyridine is corrosive to the skin and eyes and should be avoided in direct contact.
- Wear protective eyewear, gloves and protective clothing when using.
- Operate in a well-ventilated area and avoid inhaling its vapors.
- Avoid contact with strong oxidizing agents, which can cause dangerous reactions.
- Waste should be disposed of in accordance with local regulations.