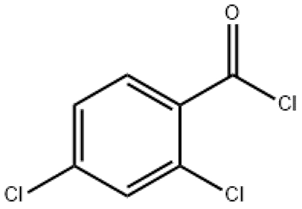
2 4-Dichlorobenzoyl chloride(CAS# 89-75-8)
| Hazard Symbols | C – Corrosive |
| Risk Codes | R34 – Causes burns R36/37 – Irritating to eyes and respiratory system. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S27 – Take off immediately all contaminated clothing. S36/37/39 – Wear suitable protective clothing, gloves and eye/face protection. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| RTECS | DM6636766 |
| FLUKA BRAND F CODES | 10-19-21 |
| TSCA | Yes |
| HS Code | 29163900 |
| Hazard Note | Corrosive |
| Hazard Class | 8 |
| Packing Group | II |
Introduction
Quality:
2,4-Dichlorobenzoyl chloride is a colorless to pale yellow liquid with a pungent odor. It is unstable at room temperature and is easily hydrolyzed and decomposed, so it should be stored under inert gas. It reacts with hydrocarbons, aromatic amines, and alcohols to form corresponding amides and esters.
Use:
2,4-Dichlorobenzoyl chloride is an important intermediate in organic synthesis and has a wide range of applications in organic synthesis reactions. It can be used in the preparation of various benzoyl chloride derivatives and other organic compounds.
Method:
2,4-Dichlorobenzoyl chloride can be obtained by chlorination of p-nitrobenzoic acid or p-aminobenzoic acid. The specific method is to react p-nitrobenzoic acid or p-aminobenzoic acid with thionyl chloride to obtain an intermediate product, and then the intermediate product is further chlorinated to finally obtain 2,4-dichlorobenzoyl chloride.
Safety Information:
2,4-Dichlorobenzoyl chloride is an organic compound that is irritating and corrosive. Precautions should be taken during use and handling, avoiding contact with the skin, eyes, and respiratory tract. Appropriate protective equipment, such as lab gloves, goggles, and protective clothing, should be worn during the procedure. Contact with combustible substances and oxidizing agents should be avoided to prevent fire or explosion. When stored and transported, it should be sealed and kept away from fire and heat sources.