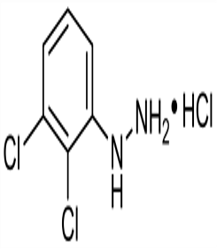
2 3-Dichlorophenylhydrazine hydrochloride(CAS# 21938-47-6)
2 3-Dichlorophenylhydrazine hydrochloride(CAS# 21938-47-6) Introduction
2,3-dichlorophenylhydrazine hydrochloride is an organic compound with the chemical formula C6H6Cl2N2 · HCl. It is a white crystalline solid, soluble in water and alcoholic solvents. The following is a description of its nature, use, preparation and safety information:
Nature:
-Appearance: White crystalline solid
-Molecular weight: 207.53g/mol
-Melting Point: 118-120 ℃
-Boiling point: 327 ℃
-Density: 1.47g/cm³
-Solubility: Soluble in water and alcohol solvents
Use:
-2,3-dichlorophenylhydrazine hydrochloride is commonly used as a reagent in organic synthesis and can be used to synthesize other organic compounds.
-It is also used as a reagent in biochemical research for the detection and analysis of specific active compounds.
-2,3-dichlorophenylhydrazine hydrochloride is also used as a raw material for pesticide intermediates and dye synthesis.
Method:
The preparation method of 2,3-dichlorophenylhydrazine hydrochloride is as follows:
First, 2,3-dichloronitrobenzene is reacted with hydrazine to generate 2,3-dichlorophenylhydrazine. Then, 2,3-dichlorophenylhydrazine is reacted with hydrochloric acid to give 2,3-dichlorophenylhydrazine hydrochloride.
Safety Information:
-2,3-dichlorophenylhydrazine hydrochloride is irritating and should avoid contact with skin, eyes and respiratory tract.
-Wear appropriate protective equipment such as lab gloves, goggles and masks when handling.
-Swallowing or inhaling an excessive amount of 2,3-dichlorophenylhydrazine hydrochloride may be harmful to health. Seek immediate medical attention in case of maladjustment.
-During storage and transportation, it should be separated from oxidants, acids and hydrochloric acid to avoid dangerous reactions.
-in the use of the process should pay attention to comply with the laboratory’s safety practices.
Nature:
-Appearance: White crystalline solid
-Molecular weight: 207.53g/mol
-Melting Point: 118-120 ℃
-Boiling point: 327 ℃
-Density: 1.47g/cm³
-Solubility: Soluble in water and alcohol solvents
Use:
-2,3-dichlorophenylhydrazine hydrochloride is commonly used as a reagent in organic synthesis and can be used to synthesize other organic compounds.
-It is also used as a reagent in biochemical research for the detection and analysis of specific active compounds.
-2,3-dichlorophenylhydrazine hydrochloride is also used as a raw material for pesticide intermediates and dye synthesis.
Method:
The preparation method of 2,3-dichlorophenylhydrazine hydrochloride is as follows:
First, 2,3-dichloronitrobenzene is reacted with hydrazine to generate 2,3-dichlorophenylhydrazine. Then, 2,3-dichlorophenylhydrazine is reacted with hydrochloric acid to give 2,3-dichlorophenylhydrazine hydrochloride.
Safety Information:
-2,3-dichlorophenylhydrazine hydrochloride is irritating and should avoid contact with skin, eyes and respiratory tract.
-Wear appropriate protective equipment such as lab gloves, goggles and masks when handling.
-Swallowing or inhaling an excessive amount of 2,3-dichlorophenylhydrazine hydrochloride may be harmful to health. Seek immediate medical attention in case of maladjustment.
-During storage and transportation, it should be separated from oxidants, acids and hydrochloric acid to avoid dangerous reactions.
-in the use of the process should pay attention to comply with the laboratory’s safety practices.
Write your message here and send it to us