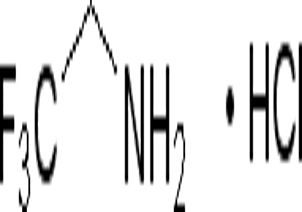2 2 2-Trifluoroethylamine hydrochloride(CAS# 373-88-6)
| Risk Codes | R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S36/37 – Wear suitable protective clothing and gloves. S24/25 – Avoid contact with skin and eyes. S36 – Wear suitable protective clothing. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| WGK Germany | 3 |
| RTECS | KS0250000 |
| FLUKA BRAND F CODES | 3-10-21 |
| TSCA | T |
| HS Code | 29211990 |
| Hazard Note | Hygroscopic/Toxic |
| Hazard Class | IRRITANT |
| Packing Group | III |
| Toxicity | LD50 unr-mus: 476 mg/kg 11FYAN 3,81,63 |
Introduction
2,2,2-Trifluoroethylamine hydrochloride, also known as TFEA hydrochloride. It is a colorless crystalline solid. The following is a detailed introduction to the properties, uses, preparation methods and safety information of TFEA hydrochloride:
Quality:
1. Appearance: colorless crystalline solid.
3. Solubility: soluble in water and common organic solvents, such as alcohols, ethers, ketones, etc.
4. Stability: Good stability, not easy to decompose.
Use:
1. As a catalyst in organic synthesis: TFEA hydrochloride is often used as a catalyst in organic synthesis reactions, such as esterification, alkylation and other reactions.
2. As a solvent: With its good solubility, TFEA hydrochloride can be used as an organic solvent, e.g. in chemical synthesis to dissolve reactants or catalysts.
3. Other applications: TFEA hydrochloride can also be used in proton conduction membranes, microelectronic devices and other fields.
Method:
The preparation method of TFEA hydrochloride is generally to react 2,2,2-trifluoroethylamine with hydrochloric acid to generate TFEA hydrochloride.
Safety Information:
1. TFEEA hydrochloride is relatively stable under normal conditions, but may decompose under high temperature and humidity conditions.
2. When using, avoid contact with strong oxidants, strong acids and other substances to prevent dangerous reactions.
3. In case of accidental contact with eyes, skin or inhalation, rinse immediately with plenty of water and seek medical advice.
4. During operation or storage, good ventilation measures should be taken to avoid inhaling dust.
5. When using TFEA hydrochloride, pay attention to follow the relevant safety operation procedures and wear appropriate protective equipment.