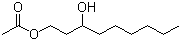
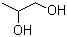
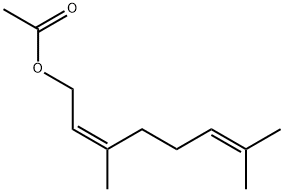
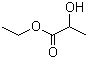
1,3-Nonanediol acetate(CAS#1322-17-4)
| WGK Germany | 2 |
1,3-Nonanediol acetate(CAS#1322-17-4) introduce
nature
Jasmine ester is an organic compound.
It is relatively stable in air, but unstable under strong acid and alkali conditions.
It is also a flammable substance and requires attention to fire prevention measures when storing and handling.
Application and synthesis method
Jasmine ester is an organic compound. It has the fragrant smell of jasmine, and is widely used as a component of spice and essence.
There are various methods for synthesizing jasmonate. Jasmine ester is usually synthesized by reacting jasmine alcohol with acetic acid. The specific steps are as follows:
Add jasmine alcohol and acetic acid into the reaction vessel;
Esterification reaction can be carried out at an appropriate temperature using acid catalysts such as sulfuric acid or zinc chloride;
After the reaction is complete, extract the obtained jasmonate by distillation or other separation methods.
Jasmine esters can also be obtained through other synthetic routes, such as using ester exchange reactions or catalytic hydrogenation reactions to convert related compounds.