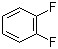
1,2-Difluorobenzene(CAS#367-11-3)
| Risk Codes | R11 – Highly Flammable R20 – Harmful by inhalation R2017/11/20 - |
| Safety Description | S7 – Keep container tightly closed. S16 – Keep away from sources of ignition. S29 – Do not empty into drains. S33 – Take precautionary measures against static discharges. S7/9 - |
| UN IDs | UN 1993 3/PG 2 |
| WGK Germany | 3 |
| RTECS | CZ5655000 |
| HS Code | 29036990 |
| Hazard Note | Flammable |
| Hazard Class | 3 |
| Packing Group | II |
Introduction
O-difluorobenzene is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of o-difluorobenzene:
Quality:
- Appearance: O-difluorobenzene is a colorless liquid or white crystal.
- Solubility: O-difluorobenzene is soluble in organic solvents such as alcohols, ethers and benzene.
Use:
- O-difluorobenzene can be used as a starting material and intermediate in organic synthesis, and is widely used in pharmaceutical, pesticide and dye fields.
- It can also be used as an additive in coatings, solvents and lubricants.
- O-difluorobenzene can also be used in the electronics industry, e.g. as a component of liquid crystal materials.
Method:
- There are two main methods for the preparation of o-difluorobenzene: the reaction of fluorine compounds with benzene and the selective fluorination reaction of fluorinated benzene.
- The reaction of fluorine compounds with benzene is common, and o-difluorobenzene can be obtained by fluorination of chlorobenzene by fluorine gas.
- Selective fluorination of fluorinated benzene requires the use of selective fluorinating reagents for synthesis.
Safety Information:
- Exposure to o-difluorobenzene may cause irritation to the skin, eyes and respiratory system, and precautions should be taken.
- Wear protective glasses, gloves and work clothes when using o-difluorobenzene, and maintain a well-ventilated environment.
- Keep away from fire and high temperatures, and store in a cool, dry place.
- Before using or handling o-difluorobenzene, read and follow the relevant safety handling guidelines.