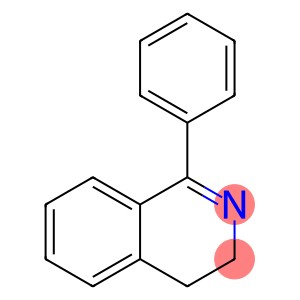
1-Phenyl-3,4-dihydroisoquinoline(CAS#52250-50-7)
1-Phenyl-3,4-dihydroisoquinoline(CAS#52250-50-7)
1-Phenyl-3,4-dihydroisoquinoline, CAS number 52250-50-7, has shown unique charm in the field of chemistry and medicine.
From the chemical essence, its molecule is cleverly combined with structural units such as phenyl group and dihydroisoquinoline ring, and this specific atomic connection mode constructs a unique electron cloud distribution, which creates its special chemical activity and stability. In appearance, it is usually presented as a solid with a certain crystalline form, the color is mostly white or off-white, and the crystal structure is regular and orderly, which is conducive to purification and refining by means of recrystallization. In terms of solubility, it shows a certain dissolution trend in common organic solvents such as ethanol and acetone, but the solubility in water is relatively low, which is closely related to the polarity of the molecule, and also provides a basis for the selection of solvent systems for subsequent separation and synthesis reactions.
In terms of pharmaceutical R&D prospects, it has potential biological activity. The chemical structure of the product is similar to that of some pharmacologically active natural products, suggesting that it may have similar targets. Preliminary exploration suggests that it may have an impact on neurological signaling pathways, and is expected to participate in the development of novel drugs for neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, by regulating abnormal neurotransmitter transmission and inhibiting the hyperapoptosis of nerve cells. At the same time, in the field of anti-tumor, the active groups in its structure may interfere with the proliferation, migration and invasion process of tumor cells, opening up new ideas for cancer treatment, of course, these are still in the early stage of laboratory research, and there is still a lot of research work to be done before clinical application.
From the perspective of industrial synthesis, the current organic chemical synthesis method is mainly relied on, starting from simple raw materials, through multi-step reactions to build a complex molecular skeleton, the process involves cyclization, substitution, condensation and other classical organic reaction types, researchers continue to optimize the reaction conditions, improve yield, reduce costs, to meet the needs of follow-up in-depth research and possible large-scale production. With the cross-integration of technologies in various fields, the all-round development of 1-Phenyl-3,4-dihydroisoquinoline is expected to accelerate, injecting new impetus into human health and scientific and technological progress.