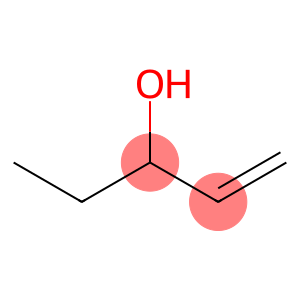
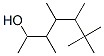
1-Penten-3-ol(CAS#616-25-1)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R10 – Flammable R37 – Irritating to the respiratory system R20 – Harmful by inhalation |
| Safety Description | S23 – Do not breathe vapour. S24/25 – Avoid contact with skin and eyes. S16 – Keep away from sources of ignition. |
| UN IDs | UN 1987 3/PG 3 |
| WGK Germany | 3 |
| TSCA | Yes |
| HS Code | 29052900 |
| Hazard Class | 3 |
| Packing Group | III |
Introduction
1-pentaen-3-ol is an organic compound. It is a naturally occurring oleic acid that is widely found in fatty acids of animals and plants. It has many important physiological and pharmacological activities.
1-pentaen-3-ol is an important precursor and regulator, which synthesizes a variety of physiologically active substances in the body, such as prostaglandins, leukotrienes, etc. It is involved in the regulation of a variety of bodily functions, including immune response, inflammatory response, platelet aggregation, and more.
There are two main preparation methods for 1-pentaen-3-ol: extraction from vegetable oil and conversion reaction. Extraction from vegetable oil is the most commonly used method to separate 1-penteno-3-ol from vegetable oil through enzymatic hydrolysis, extraction and other processes. The conversion reaction is the synthesis of 1-pentaen-3-ol through chemical reactions, such as eicosanamide and hydrogen peroxide in the presence of a catalyst.
Safety information of 1-pentaen-3-ol: Most studies have shown that it is relatively safe at certain doses. High doses or long-term large ingestions may cause adverse reactions, such as diarrhea, gastrointestinal upset, etc. It should be used with caution for certain special populations, such as pregnant women, lactating women, and infants.