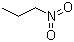1-Nitropropane(CAS#108-03-2)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | R10 – Flammable R20/21/22 – Harmful by inhalation, in contact with skin and if swallowed. |
| Safety Description | S9 – Keep container in a well-ventilated place. S24/25 – Avoid contact with skin and eyes. |
| UN IDs | UN 2608 3/PG 3 |
| WGK Germany | 1 |
| RTECS | TZ5075000 |
| TSCA | Yes |
| HS Code | 29042000 |
| Hazard Class | 3 |
| Packing Group | III |
| Toxicity | LD50 orally in Rabbit: 455 mg/kg LD50 dermal Rabbit > 2000 mg/kg |
Introduction
1-nitropropane (also known as 2-nitropropane or propylnitroether) is an organic compound. The following is a brief introduction to some of the compound’s properties, uses, preparation methods, and safety information.
Quality:
- 1-Nitropropane is a colorless liquid that is slightly flammable at room temperature.
- The compound has a pungent odor.
Use:
- 1-nitropropane is mainly used as an important intermediate in organic synthesis, which can be used to synthesize alkyl nitroketone, nitrogen heterocyclic compounds, etc.
- It can also be used as a component of explosives and propellants, industrially used in the preparation of nitro-containing explosives.
Method:
- 1-Nitropropane can be prepared by the reaction of propane and nitric acid. The reaction is usually carried out under acidic conditions, and nitric acid can react with propionic acid to obtain propyl nitrate, which can further react with propyl alcohol propionate to form 1-nitropropane.
Safety Information:
- 1-Nitropropane is a toxic substance that is irritating and corrosive. Exposure to or inhalation of its vapors may cause irritation of the eyes, skin, and respiratory tract.
- The compound should be handled in a well-ventilated area with necessary personal protective measures, such as wearing protective eyewear, gloves, and respirators.
- 1-Nitropropane should be stored in a cool, dry place, away from fire and combustible substances.
- Proper laboratory safety protocols should be followed when handling the compound.