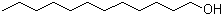1-Dodecanol(CAS#112-53-8)
| Risk Codes | R38 – Irritating to the skin R50 – Very Toxic to aquatic organisms R50/53 – Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R36/38 – Irritating to eyes and skin. R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S37/39 – Wear suitable gloves and eye/face protection S29 – Do not empty into drains. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S60 – This material and its container must be disposed of as hazardous waste. S36 – Wear suitable protective clothing. |
| UN IDs | UN 3077 9/PG 3 |
| WGK Germany | 1 |
| RTECS | JR5775000 |
| TSCA | Yes |
| HS Code | 29051700 |
| Hazard Class | 9 |
| Packing Group | III |
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg |
Introduction
Dodecyl alcohol, also known as dodecyl alcohol or dococosanol, is an organic compound. It is a solid, colorless and odorless with a special fragrance.
Dodecyl alcohol has the following properties:
2. Insoluble in water, but soluble in organic solvents such as ether and alcohol.
3. It has good stability and low volatility.
4. It has good lubricating properties and can be used as a lubricant.
The main uses of dodecyl alcohol include the following:
1. As a lubricant, it is used for the lubrication of industrial equipment and machinery.
2. As a raw material for surfactants, it can be used to prepare detergents and detergents.
3. As a solvent and diluent for dyes and inks.
4. Used as a raw material for synthetic flavors, often used in perfume and fragrance manufacturing.
The preparation method of dodecyl alcohol can be synthesized by the following methods:
1. Hydroreduction of stearate catalyzed by potassium hydroxide.
2. Through the hydrogenation reaction of dodecene.
1. Although dodecyl alcohol is a relatively safe compound, it still needs to be stored tightly sealed and avoid contact with oxygen to prevent oxidation.
2. Avoid violent reactions with strong oxidants and acids.