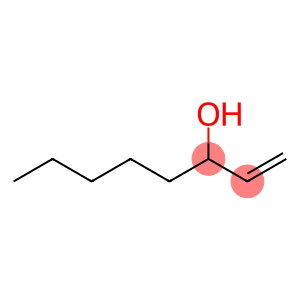
1-Butanol(CAS#71-36-3)
| Risk Codes | R10 – Flammable R22 – Harmful if swallowed R37/38 – Irritating to respiratory system and skin. R41 – Risk of serious damage to eyes R67 – Vapors may cause drowsiness and dizziness R39/23/24/25 - R23/24/25 – Toxic by inhalation, in contact with skin and if swallowed. R11 – Highly Flammable |
| Safety Description | S13 – Keep away from food, drink and animal foodstuffs. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S37/39 – Wear suitable gloves and eye/face protection S46 – If swallowed, seek medical advice immediately and show this container or label. S7/9 - S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37 – Wear suitable protective clothing and gloves. S16 – Keep away from sources of ignition. S7 – Keep container tightly closed. |
| UN IDs | UN 1120 3/PG 3 |
| WGK Germany | 1 |
| RTECS | EO1400000 |
| TSCA | Yes |
| HS Code | 2905 13 00 |
| Hazard Class | 3 |
| Packing Group | III |
| Toxicity | LD50 orally in rats: 4.36 g/kg (Smyth) |
Introduction
N-butanol, also known as butanol, is an organic compound, it is a colorless liquid with a peculiar alcoholic odor. The following is an introduction to the properties, uses, preparation methods and safety information of n-butanol:
Quality:
1. Physical properties: It is a colorless liquid.
2. Chemical properties: It can be dissolved in water and organic solvents, and is a moderately polar compound. It can be oxidized to butyraldehyde and butyric acid, or it can be dehydrated to form butene.
Use:
1. Industrial use: It is an important solvent and has a wide range of applications in the chemical industry such as coatings, inks, and detergents.
2. Laboratory use: It can be used as a solvent to induce helical protein folding, and is often used in biochemical experiments to catalyze reactions.
Method:
1. Butylene hydrogenation: After hydrogenation reaction, butene is reacted with hydrogen in the presence of a catalyst (such as a nickel catalyst) to obtain n-butanol.
2. Dehydration reaction: butanol is reacted with strong acids (such as concentrated sulfuric acid) to generate butene through dehydration reaction, and then butene is hydrogenated to obtain n-butanol.
Safety Information:
1. It is a flammable liquid, avoid contact with the fire source, and keep away from open flames and high temperature environments.
3. It has a certain toxicity, avoid direct contact with the skin and eyes, and avoid inhaling its vapor.
4. When storing, it should be stored in a closed space, away from oxidants and fire sources, and stored at room temperature.