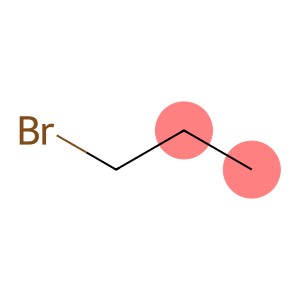
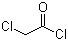
1-Bromopropane(CAS#106-94-5)
| Risk Codes | R60 – May impair fertility R11 – Highly Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. R48/20 - R63 – Possible risk of harm to the unborn child R67 – Vapors may cause drowsiness and dizziness |
| Safety Description | S53 – Avoid exposure – obtain special instructions before use. S45 – In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) |
| UN IDs | UN 2344 3/PG 2 |
| WGK Germany | 2 |
| RTECS | TX4110000 |
| FLUKA BRAND F CODES | 8 |
| TSCA | Yes |
| HS Code | 29033036 |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | LD50 orally in Rabbit: > 2000 mg/kg LD50 dermal Rat > 2000 mg/kg |
Introduction
Propane bromide is an organic compound. The following is an introduction to the properties, uses, preparation methods and safety information of propylvane bromide:
Quality:
Propane bromide is a colorless, volatile liquid. It is insoluble in water but soluble in common organic solvents such as alcohols, ethers, etc.
Use:
Propane bromide has a wide range of applications in the field of organic synthesis. It can be used as a reagent and intermediate for the synthesis of other organic compounds.
Method:
The main method of preparing propyl bromide is by reacting propane with hydrogen bromide. This reaction takes place at room temperature, often using dilute sulfuric acid as a catalyst. The reaction equation is: CH3CH2CH3 + HBr → CH3CH2CH2Br + H2.
Safety Information:
Propane bromide is a toxic, irritating compound. Contact with the skin and eyes can cause irritation, and inhalation of high concentrations of propylene bromoide vapor can cause dizziness, nausea, and lung damage. Long-term or frequent exposure to propylvane bromide can be harmful to the nervous system, liver and kidneys. When using and storing propylene bromide, contact with ignition sources should be avoided and good ventilation conditions should be maintained. Appropriate personal protective equipment should be worn during laboratory operations and safe operating procedures should be followed.