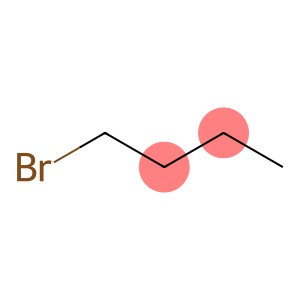
1-Bromobutane(CAS#109-65-9)
| Risk Codes | R11 – Highly Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R10 – Flammable |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S60 – This material and its container must be disposed of as hazardous waste. S37/39 – Wear suitable gloves and eye/face protection |
| UN IDs | UN 1126 3/PG 2 |
| WGK Germany | 2 |
| RTECS | EJ6225000 |
| TSCA | Yes |
| HS Code | 29033036 |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | LD50 orally in Rabbit: 2761 mg/kg |
Introduction
1-Bromobutane is a colorless liquid with a peculiar pungent odor. Bromobutane has moderate volatility and vapor pressure, is soluble in organic solvents, and insoluble in water.
1-Bromobutane is widely used as a brominating reagent in organic synthesis. It can be used as a substrate for brominated reactions such as nucleophilic substitution reactions, elimination reactions, and rearrangement reactions. It can also be used as an industrial solvent, for example in petroleum extraction to remove wax from crude oil. It is irritating and toxic, and must be handled with caution and equipped with appropriate precautions when used.
A common method for the preparation of 1-bromobutane is by the reaction of n-butanol with hydrogen bromide. This reaction is carried out under acidic conditions to produce 1-bromobutane and water. The specific reaction conditions and the choice of catalyst will affect the yield and selectivity of the reaction.
It is irritating to the skin and eyes, and inhaling too much can cause breathing difficulties and neurological damage. It must be performed in a well-ventilated area and with protective gloves, goggles, and respirators worn. When storing and handling, keep away from ignition sources and oxidants to prevent the risk of fire and explosion.