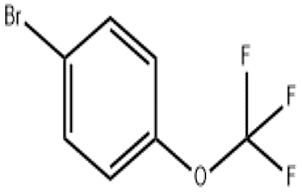1- Bromo-4-(trifluoromethoxy)benzene(CAS# 407-14-7)
| Risk Codes | R22 – Harmful if swallowed R36/37/38 – Irritating to eyes, respiratory system and skin. R43 – May cause sensitization by skin contact R51/53 – Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
| Safety Description | S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36/37 – Wear suitable protective clothing and gloves. S61 – Avoid release to the environment. Refer to special instructions / safety data sheets. S37/39 – Wear suitable gloves and eye/face protection S36 – Wear suitable protective clothing. |
| UN IDs | UN 3082 9/PG 3 |
| WGK Germany | 1 |
| HS Code | 29093090 |
| Hazard Class | IRRITANT |
| Toxicity | LD50 orally in Rabbit: > 2500 mg/kg |
Introduction
Bromotrifluoromethoxybenzene (BTM) is an organic compound. The following is an introduction to the nature, use, manufacturing method, and safety information of BTM:
Quality:
- Appearance: Bromotrifluoromethoxybenzene is a colorless or light yellow liquid.
- Odor: Has a special smell.
- Solubility: Can be dissolved in organic solvents such as ethanol and ether.
Use:
Bromotrifluoromethoxybenzene is mainly used as a reaction reagent in organic synthesis. It can be used as a phenyl brominating agent, fluorinating reagent, and methoxylating reagent.
Method:
The preparation method of bromotrifluoromethoxybenzene is generally obtained by the reaction of bromotrifluorotoluene and methanol. For the specific preparation process, please refer to the manual of organic synthesis chemistry or the relevant literature of organic chemistry.
Safety Information:
- Bromotrifluoromethoxybenzene is irritating and may cause irritation and burns in contact with the skin and eyes.
- Avoid inhaling vapours or gases from the substance and keep it well ventilated.
- Wear protective gloves, glasses and protective clothing when in use.
- This compound should be stored in an airtight container, away from fire and heat sources, and avoid contact with oxidants and strong acids.