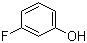
1 1 1-Trifluoroacetone(CAS# 421-50-1)
| Risk Codes | R12 – Extremely Flammable R36/37/38 – Irritating to eyes, respiratory system and skin. |
| Safety Description | S16 – Keep away from sources of ignition. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S29 – Do not empty into drains. S33 – Take precautionary measures against static discharges. S36 – Wear suitable protective clothing. S7/9 - S9 – Keep container in a well-ventilated place. S37/39 – Wear suitable gloves and eye/face protection S39 – Wear eye / face protection. |
| UN IDs | UN 1993 3/PG 1 |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 19 |
| TSCA | T |
| HS Code | 29147090 |
| Hazard Note | Flammable/Lachrymatory |
| Hazard Class | 3 |
| Packing Group | I |
Introduction
1,1,1-Trifluoroacetone. The following is an introduction to its nature, use, preparation method and safety information:
Quality:
1,1,1-trifluoroacetone is a flammable liquid with a spicy and sweet taste. It is highly chemically stable, is not easily decomposed by acids, alkalis or oxidants, and is not easily hydrolyzed. It has good solubility and is soluble in a variety of organic solvents such as alcohols, ethers, and ketones.
Use:
1,1,1-Trifluoroacetone has a wide range of uses in industry. It is an important solvent that can be used in areas such as coatings, cleaners, degreasers, and gas sealants. It can also be used as a swelling agent for polyurethane, polyester and PTFE, as well as as a plasticizer and flame retardant for coatings.
Method:
The preparation of 1,1,1-trifluoroacetone is mainly made by the reaction of fluorinated reagent with acetone. A common method is to use ammonium bifluoride (NH4HF2) or hydrogen fluoride (HF) to react with acetone in the presence of a catalyst to produce 1,1,1-trifluoroacetone. This reaction process needs to be carried out under strict control because hydrogen fluoride is a toxic gas.
Safety Information:
1,1,1-Trifluoroacetone is a flammable liquid that can explode when exposed to an open flame or high temperature. It has a low flash point and autoignition temperature, and needs to be handled and stored properly, away from ignition and hot objects. Appropriate protective equipment such as protective eyewear, gloves and protective clothing should be worn when in use. It should be ensured to operate in a well-ventilated area and avoid inhaling its vapors as it can cause damage to the human body. In case of contact with skin or eyes, rinse immediately with plenty of water and seek medical assistance.


![4-[2-(3 4-dimethylphenyl)-1 1 1 3 3 3-hexafluoropropan-2-yl]-1 2-dimethylbenzene(CAS# 65294-20-4)](https://www.xinchem.com/uploads/4234dimethylphenyl111333hexafluoropropan2yl12dimethylbenzene.png)