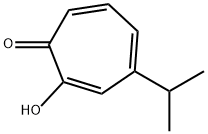
β-thujaplicin(CAS# 499-44-5)
| Hazard Symbols | Xn – Harmful |
| Risk Codes | 22 – Harmful if swallowed |
| Safety Description | 36 – Wear suitable protective clothing. |
| WGK Germany | 3 |
| RTECS | GU4200000 |
Introduction
Hinokiol, also known as α-terpene alcohol or Thujanol, is a natural organic compound that belongs to one of the components of turpentine. Hinoylol is a colorless, transparent liquid with a fragrant pine flavor.
Hinokiol has a variety of uses. It is widely used in the perfume and fragrance industry to add fragrance and fragrance to products. Secondly, juniper alcohol is also used as a fungicide and preservative, and is often used in the preparation of disinfectants and fungicides.
There are several ways to prepare juniperol. Usually, it can be extracted by distillation of volatile oils from juniper leaves or other cypress plants, and then separated and purified to obtain juniperol. Hinoki alcohol can also be synthesized by chemical synthesis.
Safety information of juniperol: It is less toxic and is generally considered safe. As an organic compound, it still needs to be handled and stored correctly. Avoid contact with skin and eyes, and rinse immediately with water in case of accidental contact. It should be kept away from open flames and high temperatures, and stored in a cool, dry place.